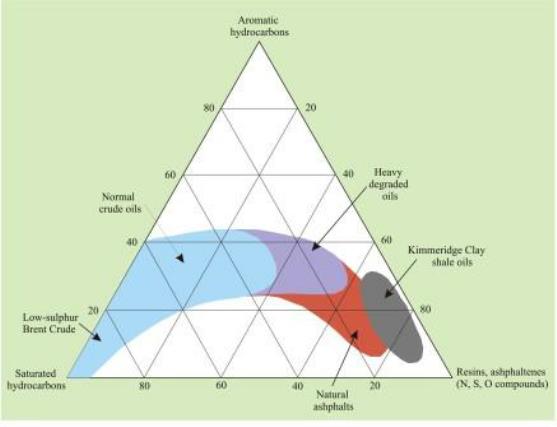
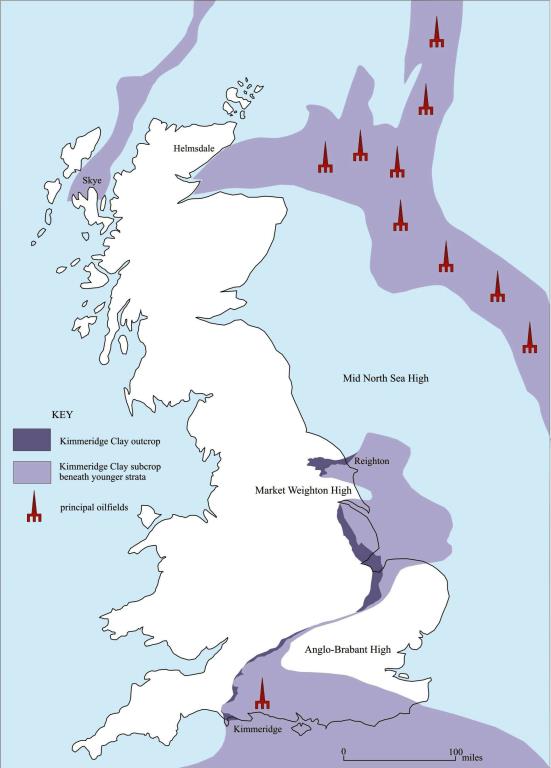


The Kimmeridge Clay beneath the North Sea was deposited in relatively deep, fault-bounded marine basins. These allowed oxygen deficient (disaerobic and anaerobic) conditions to develop on the sea floor that were conducive to the preservation of organic material, largely the remains of planktonic algae (e.g. dinoflagellates) and animals. In the complex geochemistry in the sediment, the biogenic lipids (fats, waxes, sterols etc) break down and their derivatives polymerise to produce kerogens, complex lattice-like compounds with molecular weights in the thousands. When sufficiently deeply buried for them to be heated to temperatures of c. 60° to c. 120° C (the ‘oil window’) by the geothermal gradient the kerogens are thermally broken down to produce oil.
The Blackstone was used in the Bronze Age and by the Romans to make ornamental vases and bowls, and probably also as a fuel. From medieval times onwards it was used as a fuel under the name Kimmeridge Coal. It burns with a sooty, foul smelling flame on account of its high sulphur content, and leaves behind large quantities of ash. In the 17th century it was used as the heat source to make alum from shale and glass from sand, and to extract salt from seawater. It was used domestically until the 1930s. Accounts of rural Dorset by Victorian gentlemen travellers describe evil smelling peasants crouching around smoky fires in their sooty hovels.
The most commercially desirably naturally occurring crude oils are those that are composed almost entirely of hydrogen and carbon compounds. These are the cheapest to fractionate to produce the high-purity fuels needed for petrol, diesel and aviation engines. Ideally, the sulphur content of the crude oil needs to be low (c. 0.5%: known as a ‘sweet crude’). Most fuels have sulphur contents of 5 to 10 ppm with (in Europe) a maximum permitted content of 50 ppm (0.0005%). Shale oils derived from the Kimmeridge Clay and Lias typically have sulphur contents in the range 5 to 8%. This makes them expensive to purify, and has been quoted as the principal cause of failure of the proposed Norfolk and Somerset oil-shale industries.
Retorting oil shales to produce shale oil, and their direct burning as a fuel, produces sulphurous gases and large volumes of spent shale. The shale oils, spent shale and the aqueous distillate from the retorting or from burning may contain low concentrations of biologically active compounds including carcinogens. As a result, most of the waste materials produced by commercial oil-shale working throughout the world has been left as abandoned tips. In theory, the high sulphur contents in shale oils, such as those derived from Jurassic oil shales in the UK, could be converted into commercially valuable products such as ammonium sulphate, but the technical difficulties of doing so are considerable.
Lothians oil shale
Kimmeridge Clay Blackstone
spent oil shale waste